The density of water is maximum at 4 0 C and the volume reaches a minimum. Note that the density of pure water is defined to be 1 gram per cubic centimeter or gml.

Metrodorus Ocean Sea And Ocean Ocean Waves
4-5 intensely colored ice cubes per group make these ahead of time Salt.

. When you mix two water masses temperature and salinity mix linearly. Students use two different methods to determine the densities of a variety of materials and objects. Which of these statements would best explain this fact.
Beyond this temperature water behaves like a usual liquid ie. Since water has a density of 1 gram per milliliter 1 gmL or 1 gcm3 a material or object with a density higher than one will sink and anything with a density lower than one will float1 As one can see from the Density Equation d mV there are two ways of changing the density of an object. 2 populations can be the same size but have different densities due to the size of that arearegioncountry.
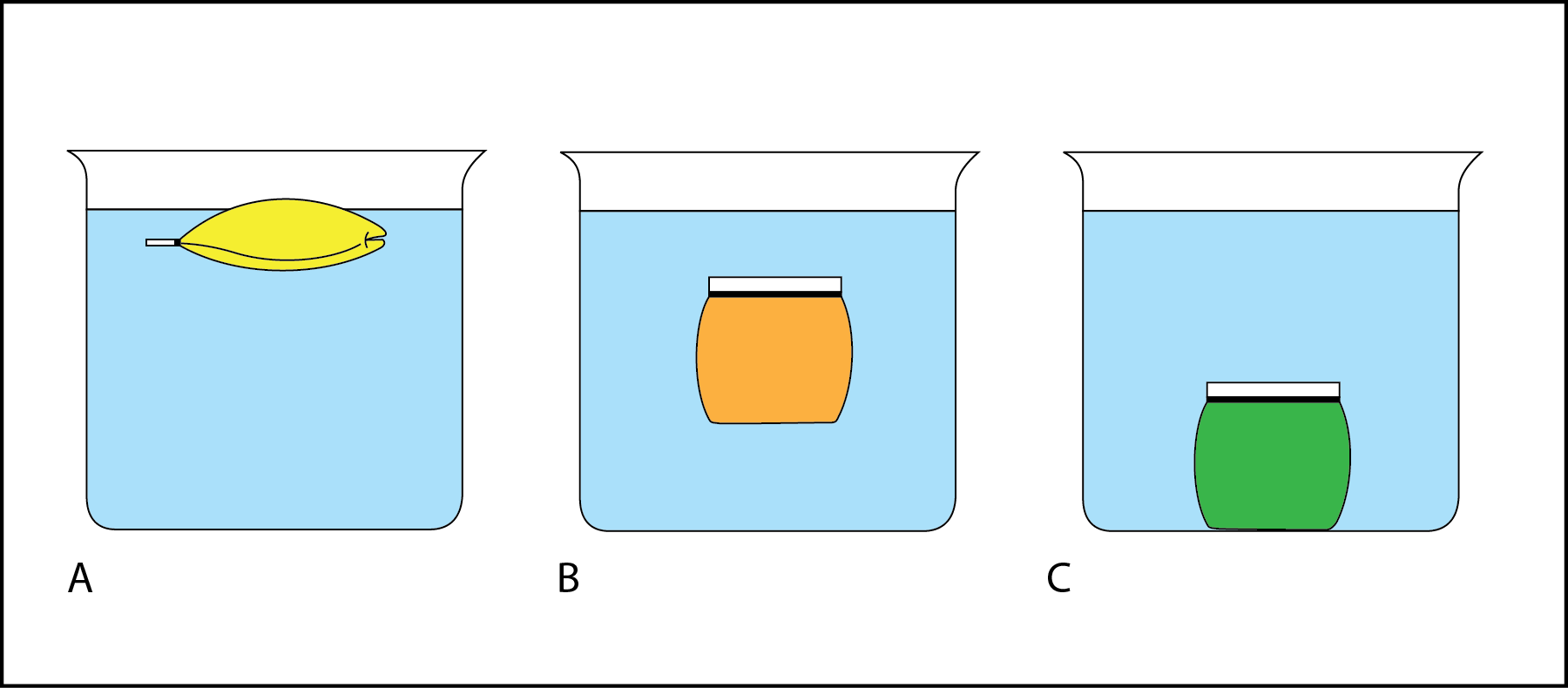
However when placed in water the green tomato floats and the red one sinks. The density of water increases from 0 0 C to 4 0 C unlike usual liquids. Just like a solid the density of a liquid equals the mass of the liquid divided by its volume.
Two samples of water can have different densities. The density and volume graphs with temperature are shown. 1 clear food storage box approximately 6 in square per group.
He poured the substances in separate beakers of water and stirred each one using a glass rod. A substance with larger lighter atoms that are farther apart is going to have a lower density. However because of the non-linearity of the relation of density as a function of temperature and salinity the density of the resulting mixture is actually greater than either initial water parcel.
The two objects have different densities. B the two samples are of different volumes. It has 2 different densities because some are regular and salt water mix together and that makes it have 2 different densities Wiki User 2008-10-29 013101.
The second is the water displacement method used to determine the volumes of irregularly shaped objects. A liquid will sink if it is more dense than the liquid it is placed in. Its no coincidence that water has a density of 1.
Plz answer these questions. Calculate the density for each metal slug in two ways. Water chemical formula H 2 O is an inorganic transparent tasteless odorless and nearly colorless chemical substance which is the main constituent of Earths hydrosphere and the fluids of all known living organisms in which it acts as a solventIt is vital for all known forms of life even though it provides no calories or organic nutrientsIts chemical formula H 2 O indicates.
So 1g1cm 3 1 gcm 3 giving water its easy-to-remember density. Density is mass divided by volume ρmv and water was used as the basis for establishing the metric unit of mass which means a cubic centimeter 1cm 3 of water weighs one gram 1g. A liquid will float if it is less dense than the liquid it is placed in.
Anomalous Expansion of Water. How might a substance such as water have two different densities. Which of these statements would best explain this fact.
It has 2 different densities because some are regular and salt water mix together and that makes it have 2 different densities. A The two samples are of different masses. Key Ideas About the Density of Liquids.
1000 kgm 3. The density of a liquid determines whether it will float on or sink in another liquid. C the two samples are in different states solid liquid gas.
Density is defined as massvolume and is usually expressed as gmL or gcm3 in the metric system. When the mass is more then its gravitational attraction will be higher Compared to other lighter atomic mass liquidsbut these gravitational attraction has to be too small to be noticeable. For instance if there are 50 grams of a given substance within a cubic centimeter that units density is 50gmm3.
If water was most dense at the freezing point then in winter the very cold water at the surface of lakes would sink the lake could freeze from the bottom up. The volume of a liquid can be measured directly with a graduated cylinder. That is if the two parcels are the same size the resulting salinity and temperature are just the average of the two.
Unlike most substances water is denser as a liquid than as a solid. A the two samples are of different masses. B The two samples are of different volumes.
A green tomato also has a mass of 456 g. If a country 1 had a population of 60000000 people and was 10000 square kilometres. Explain how two tomatoes with the same mass can have two different densities.
The maximum density of water is 1000 kgm 3. C The two samples are in different states solid liquid gas. That is density p is equal to total mass M divided by total volume v.
Extend Have students explain on the molecular level why two blocks of different materials that have the same mass can have different densities. Common units for the measurement of density include grams g milliliters ml or grams per cubic centimeter. Which of the two is denser.
This formula can be used to determine the density of any substance. Blue food coloring red or green works too Ice cube trays. Volume of water stopper slug minus volume of water stopper 3.
A certain red tomato has a mass of 456 g. Also pure water is less dense than seawater so fresh water can float on top of salt water mixing at the interface. Since water at about 39F 4C is more dense than water at 32F 0C in lakes and other water bodies the denser water sinks below less-dense water.
Mass can be measured indirectly using a scale and a graduated cylinder or other container. A consequence is that ice floats on water. If you have 10 ml of mercury and 10 ml of water the mercury will have a.
Since density is a characteristic property of a substance each liquid has its own characteristic density. Density means that if you take two cubes of the same size made out of different materials and weigh them they usually wont weigh the same. The two objects have different temperatures.
Either changing the mass or changing the volume. As an example mercury is a liquid metal whose density is 135 times that of water. How might these factors work together to cause a substance to have a low density.
And Another Country 2 had a population of 60000000 people and was 5000 square kilometres then country 2 would have a bigger density as more people. The first method involves direct measurement of the volumes of objects that have simple geometric shapes. Density of water lbft 3.
It also means that a huge cube of Styrofoam can weigh. Plz answer these questions fast. The volume of water displaced is represented in mL and can be calculated by.
We know that different liquids have different densities and it is mass over volume. The two objects are composed of the same substances. 12 liter of refrigerated water per group.
Mario performed an experiment to investigate how certain substances dissolve in water. 4 sticks of classroom modeling clay per group. Two samples of water can have different densities.

Density Sink And Float For Liquids Chapter 3 Density Middle School Chemistry
Density Temperature And Salinity Manoa Hawaii Edu Exploringourfluidearth

What Is The Density Of Water Factors Experiment Temperature Scales Faqs
0 Comments